13
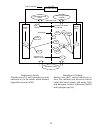
Salt water contains four kinds of ions; sodium ions (Na
+
), chlorine ions (Cl
-
), hydrogen ions
(H
+)
and hydroxide ions (OH
-
).
NaCl + H
2
O → Na
+
+ Cl
-
+ H
+
+ OH
-
(Mix water and salt) Salt water (4 kinds of ions)
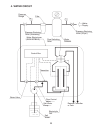
When two electrodes are inserted into salt water and voltage is applied:
Negative ions (Cl
-
) are drawn to the anode, and
Positive ions (Na
+
) are drawn to the cathode.
At the anode, hydrogen chloride (HCl) and hypochlorous acid (HOCl) are generated.
2Cl
-
+ H
2
O → HCl + HOCl + 2e
-
Electrons (2e
-
) are emitted to the anode, which means the acidic water (HCl + HOCl)
causes
oxidization. [As electrons are emitted, the oxidization/reduction potential becomes positive
(+mV).]
Chlorine ions also emit electrons and become chlorine gas (Cl
2
).
2Cl
-
→ Cl
2
+ 2e
-
(Cl
2
= chlorine gas)
At the cathode, sodium hydroxide (NaOH) and hydrogen gas (H
2
) are generated.
Na
+
+ H
2
O + H
+
+ 2e
-
→ NaOH + H
2
Electrons (2e
-
) are received from the cathode, which means the alkali water (NaOH)
causes reduction. [As electrons are received, the oxidization/reduction potential becomes
negative (-mV).]