11
II. TECHNICAL INFORMATION
1. PRINCIPLE OF ELECTROLYSIS
Water (H
2
O) we use in our daily life has a mysterious power. Adding a small amount of salt
(NaCl) to water (H
2
O) and electrolyzing it with special electrodes will generate "electrolytic
oxidizing water (acidic water)" with strong oxidizing effects and "electrolytic reducing water
(alkaline water)" with strong reducing effects. Here we explain this electrolysis process and
the meaning of such terms as "pH" and "oxidization/reduction" which may sound unfamiliar.
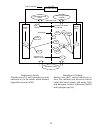
Electrolysis Process - See the diagram on the following page for the electrolysis
mechanism inside the electrolytic cell.
1) Electrolysis with a higher salt concentration around 5 - 20% is apt to generate chlorine
gas (Cl
2
) at the anode. The electrolyzer with a lower salt concentration around 0.07 -
0.15% is apt to generate hypochlorous acid (HOCl) at the anode.
2) At the anode, oxidization will generate hypochlorous acid (HOCl) and chlorine gas (Cl
2
).
3) At the cathode, reduction will generate hydrogen gas (H
2
) and sodium hydroxide (NaOH).
Oxidization/Reduction - Oxidization and reduction occur a the same time, while electrons
are transferred.
1) Oxidization - Reaction of a substance to emit electrons.
2) Reduction - Reaction of a substance to receive electrons.
Oxidization/Reduction Potential - Degree of liability to oxidization and reduction,
indicated in "mV".
1) Positive potential - An oxidizing agent (= a substance capable of oxidizing other
substances) is contained. The higher potential shows the higher tendency to oxidize
other substances.
2) Negative potential - An reducing agent (= a substance capable of reducing other
substances) is contained. The lower potential shows the higher tendency to reduce
other substances.
pH - Concentration index of hydrogen ions. pH7 means neutrality, the higher pH alkalinity,
and the lower pH acidity.