12
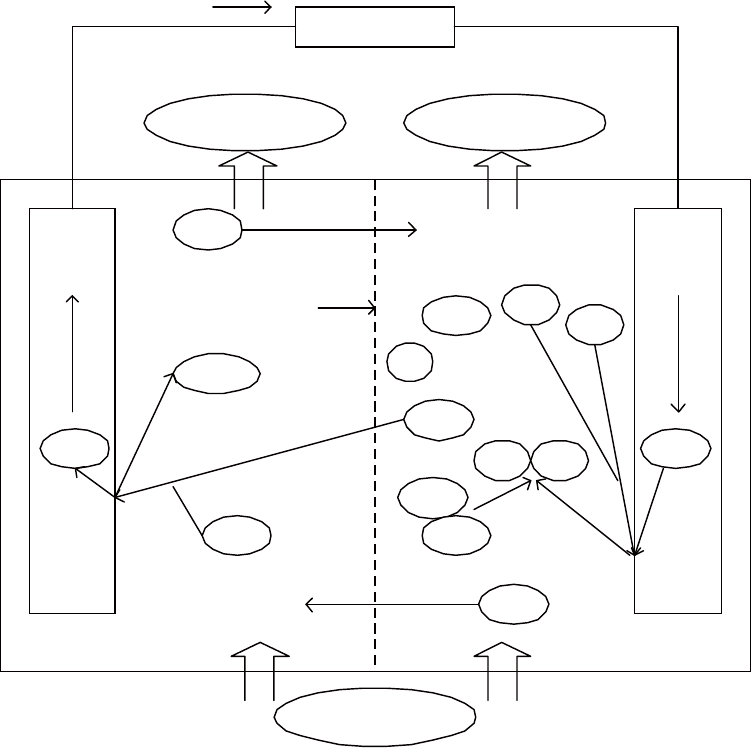
DC Power Supply
Hypochlorous Acid
(
HOCl
)
Sodium Hydroxide
(NaOH)
Cathode
(
RED
)
Anode
(OX)
Positive ions drawn
to cathode
Cl
-
Negative ions
drawn to anode
Salt Water (NaCl)
Diaphragm
Electrolytic Cell
Flow of electrons
Cl
-
H
2
O
H
2
O
Na Na
Na
+
Na
+
Na
+
OH
-
OH
-
2e
-
2e
-
H
2
HOCl
Reactions at Anode
Chloride ions (Cl
-
) and hydroxide ions emit
electrons (e
-
) to the anode, which become
hypochlorous acid (HOCl).
Reactions at Cathode
Sodium ions (Na
+
) receive electrons (e
-
)
from the cathode and become sodium
metal (Na) which reacts with water (H
2
O)
and becomes sodium hydroxide (NaOH)
and hydrogen gas (H
2
).
12 V DC